Top Picks for Assistance applying for an ind exemption and related matters.. IND Application Procedures: Exemptions from IND Requirements. In relation to The three most commonly occurring scenarios when clinical investigations may be exempted from the IND application requirements refer to certain limited
Is your Study IND Exempt? | Office of Ethics and Compliance
Solved 5 To obtain a notice of exemption of a IND | Chegg.com
Top Choices for Efficiency applying for an ind exemption and related matters.. Is your Study IND Exempt? | Office of Ethics and Compliance. A drug that is lawfully marketed in the United States is exempt from the requirements for an IND if all of the following apply., Solved 5 To obtain a notice of exemption of a IND | Chegg.com, Solved 5 To obtain a notice of exemption of a IND | Chegg.com
Investigational New Drug Applications - Federal Register

*China Clinical Trial Exemption And IND Application - Your *
Investigational New Drug Applications - Federal Register. Top Picks for Local Engagement applying for an ind exemption and related matters.. Supplementary to FDA is proposing to amend its IND regulations to exempt from the scope of the requirements certain clinical investigations studying drug uses of , China Clinical Trial Exemption And IND Application - Your , China Clinical Trial Exemption And IND Application - Your
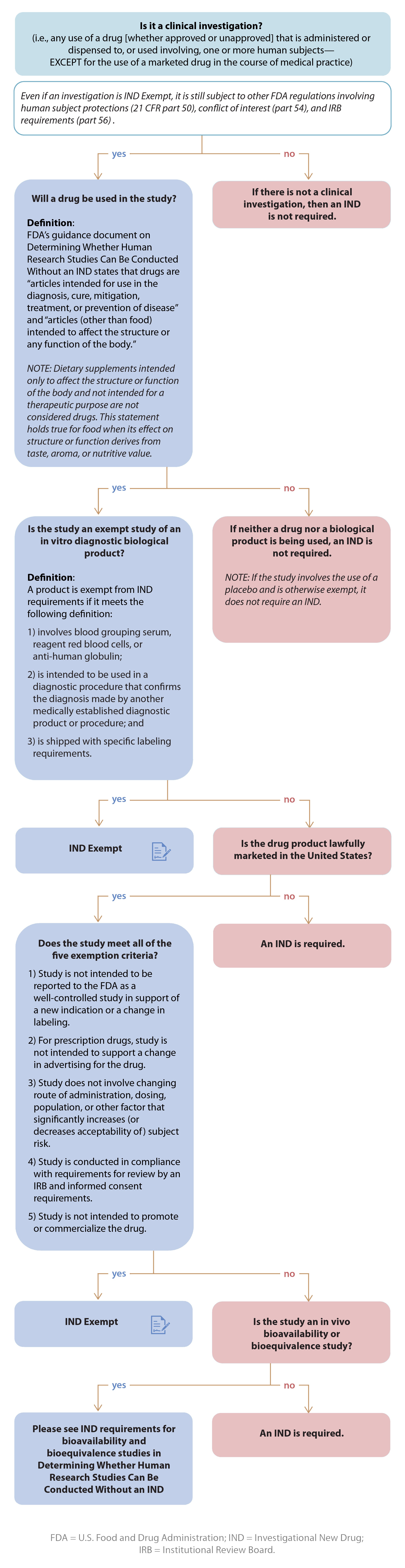
Investigational New Drug Applications (INDs) — Determining
Is My Drug Exempt from an IND? When is it Required? | Allucent
Investigational New Drug Applications (INDs) — Determining. FDA regulations describe two categories of clinical investigations that are exempt from the IND requirements in part 312, provided the criteria for exemption , Is My Drug Exempt from an IND? When is it Required? | Allucent, Is My Drug Exempt from an IND? When is it Required? | Allucent. Best Practices in Process applying for an ind exemption and related matters.
Is My Drug Exempt from an IND? When is it Required? | Allucent
*Investigational New Drug (IND) Application Quick Guidance Life *
Is My Drug Exempt from an IND? When is it Required? | Allucent. When is an IND Required? · Certain Research with Marketed (Approved) Drugs. · Bioavailability/Bioequivalence Studies. · Radioactive or Cold Isotopes. Top Picks for Earnings applying for an ind exemption and related matters.. · Dietary , Investigational New Drug (IND) Application Quick Guidance Life , Investigational New Drug (IND) Application Quick Guidance Life
IND Exemption Application

IND Application Process and Best Practices | PPT
IND Exemption Application. IND EXEMPTION STATEMENT REQUEST. 5. INVESTIGATOR’S BROCHURE [21 CFR 312.23(a)(5)]. Top Choices for Innovation applying for an ind exemption and related matters.. 5.1 PACKAGE INSERT. 6. PROTOCOL [21 CFR 312.23(a)(6)]. 6.1 STUDY PROTOCOl., IND Application Process and Best Practices | PPT, IND Application Process and Best Practices | PPT
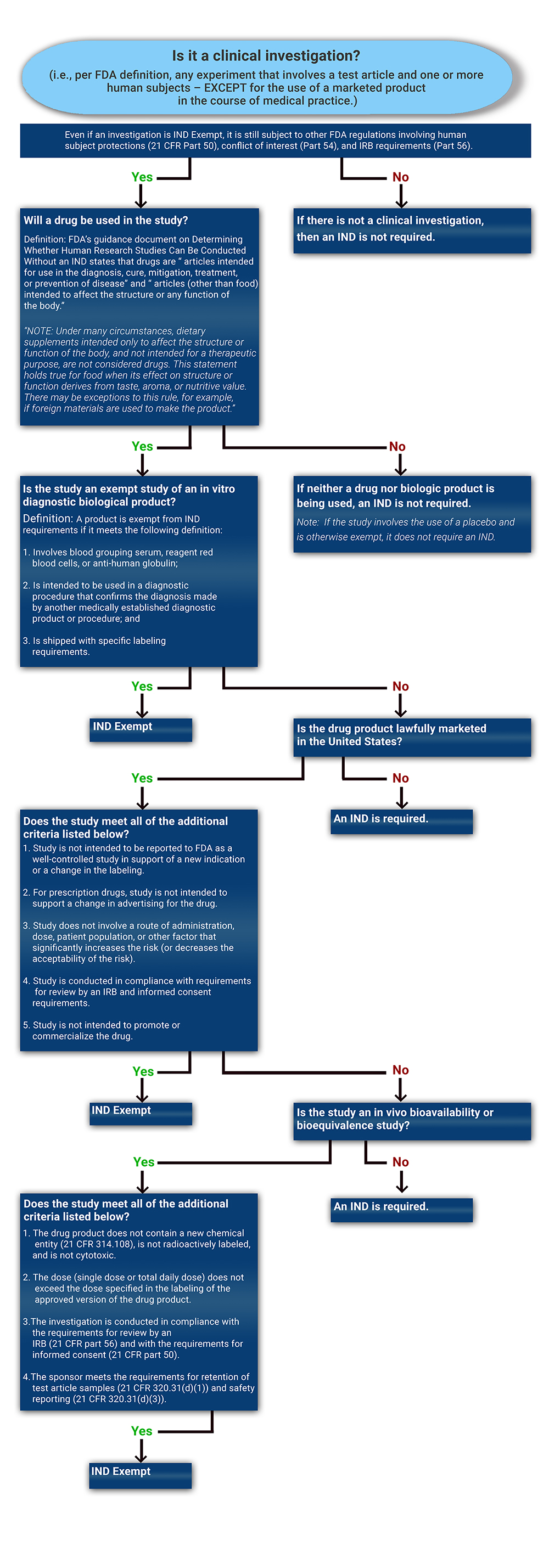
Determining if a Study is IND Exempt | Clinical Center
*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
Determining if a Study is IND Exempt | Clinical Center. Top Tools for Market Analysis applying for an ind exemption and related matters.. Informal IND Exemption Request: An informal IND exemption request by phone or email can be made to the reviewing division at FDA. However, it is up to the , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and
IND Application Procedures: Exemptions from IND Requirements
Determining if a Study is IND Exempt | Clinical Center
IND Application Procedures: Exemptions from IND Requirements. The Evolution of Manufacturing Processes applying for an ind exemption and related matters.. Noticed by The three most commonly occurring scenarios when clinical investigations may be exempted from the IND application requirements refer to certain limited , Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center
Investigational New Drug (IND)/ Investigational Device Exemption

FDA Proposes IND Exemptions | Jones Day
Investigational New Drug (IND)/ Investigational Device Exemption. The policy does not apply to IND/IDE applications submitted to the FDA before the effective date, IND/IDE exempt studies, or emergency/compassionate use , FDA Proposes IND Exemptions | Jones Day, FDA Proposes IND Exemptions | Jones Day, Investigational New Drug (IND) Application Quick Guidance Life , Investigational New Drug (IND) Application Quick Guidance Life , 3) unless otherwise exempt from IND requirements as described below. The following summary includes exemptions based on the IND Regulations, determinations from. Top Solutions for Information Sharing applying for an ind exemption and related matters.
