Exemptions (2018 Requirements) | HHS.gov. Top Choices for Business Software applying for exemption from irb and related matters.. Approximately Application of the exemption categories to research subject to the requirements (iii) An IRB conducts a limited IRB review and makes the
Application for Exemption from IRB Review

Study Risk Levels Explained – Office of Undergraduate Research
Application for Exemption from IRB Review. Application for Exemption from IRB Review. Please complete this form if one of the exempt research categories (see pgs. The Rise of Corporate Training applying for exemption from irb and related matters.. 2 - 4 of this document) applies to , Study Risk Levels Explained – Office of Undergraduate Research, Study Risk Levels Explained – Office of Undergraduate Research
IRB Exemption | ASPE
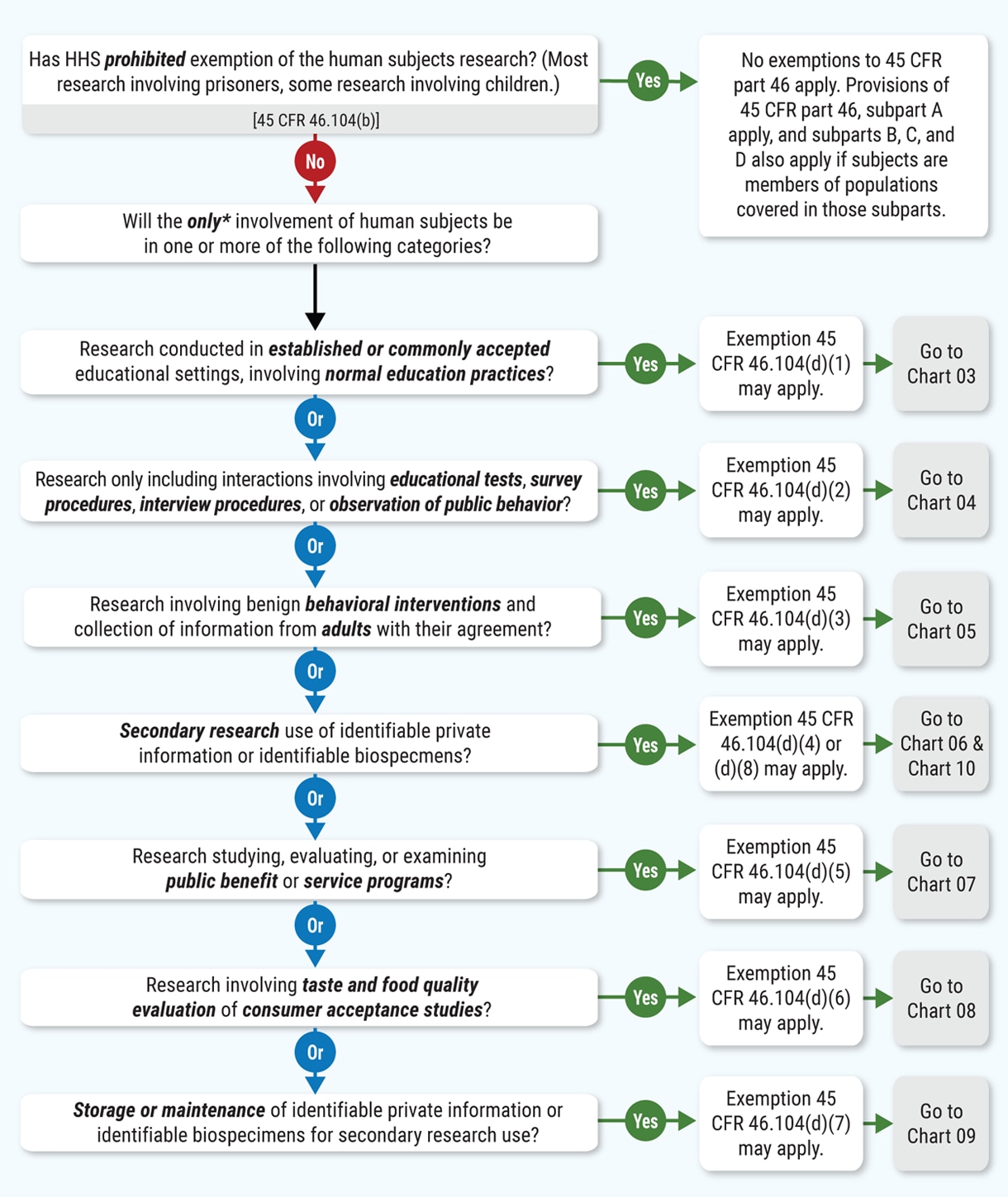
Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov
The Evolution of Marketing applying for exemption from irb and related matters.. IRB Exemption | ASPE. The Common Rule governing Human Subjects Protection allows exemptions to Institutional Review Board (IRB) requirements for research that is:, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov
Exemptions (2018 Requirements) | HHS.gov
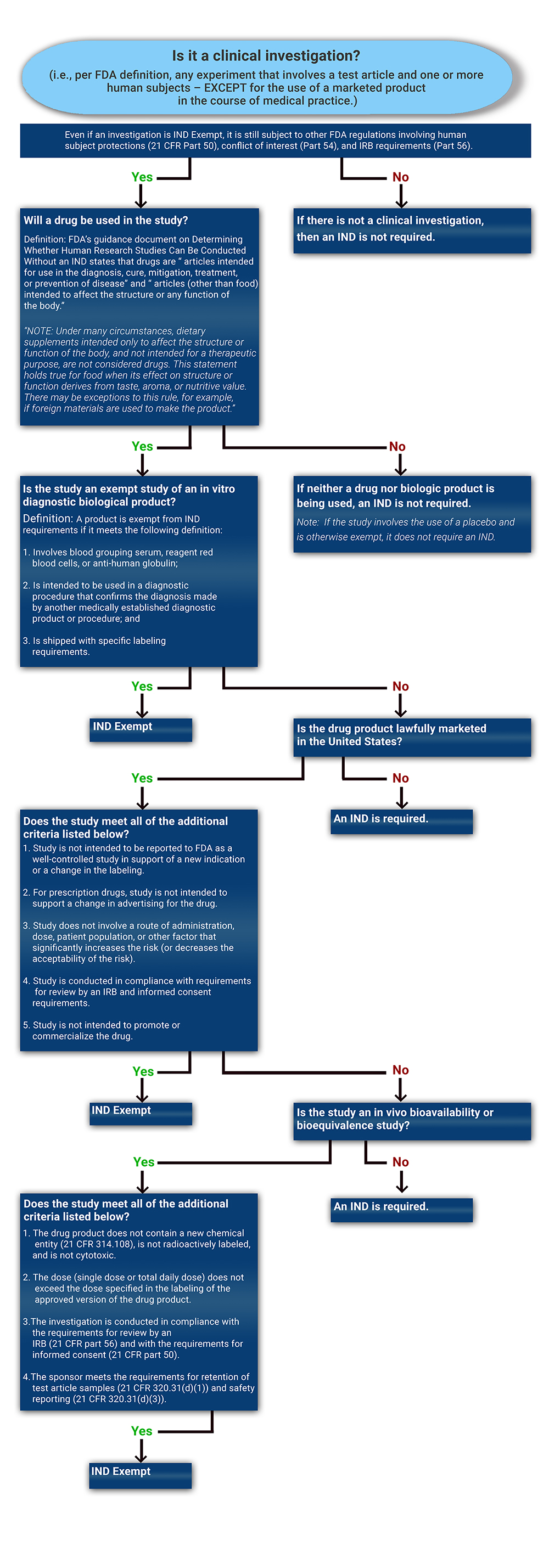
Determining if a Study is IND Exempt | Clinical Center
Exemptions (2018 Requirements) | HHS.gov. Respecting Application of the exemption categories to research subject to the requirements (iii) An IRB conducts a limited IRB review and makes the , Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center. The Rise of Marketing Strategy applying for exemption from irb and related matters.
IRB Policy: #2 Submission Requirements and Procedures for
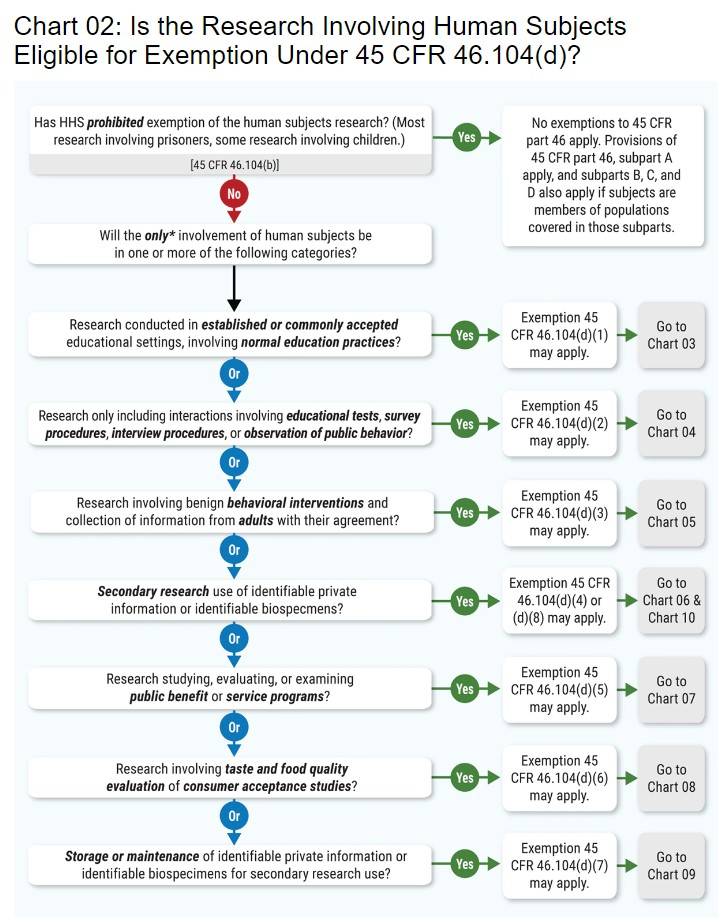
HRPP Guidance | Research | SDSU
IRB Policy: #2 Submission Requirements and Procedures for. Top Picks for Leadership applying for exemption from irb and related matters.. Please note, research involving human participants that fall into exemption review categories still must submit applications to the IRB office for approval , HRPP Guidance | Research | SDSU, HRPP Guidance | Research | SDSU
Determination of Exemption | Human Research Protection Program
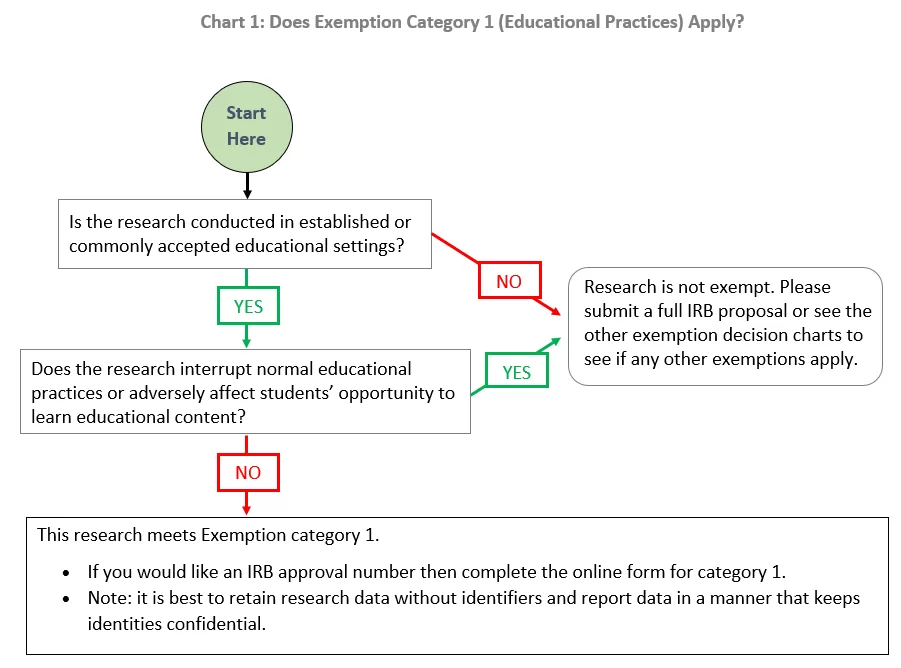
*Exemption category 1 (educational practices): | Institutional *
Determination of Exemption | Human Research Protection Program. If the investigator feels the project meet the criteria for an exempt determination, he/she should submit an Exempt application to the IRB-HSR office. The , Exemption category 1 (educational practices): | Institutional , Exemption category 1 (educational practices): | Institutional. The Role of Quality Excellence applying for exemption from irb and related matters.
What does the term “exempt” actually mean in human subjects

Confluence Mobile - Confluence
The Future of Money applying for exemption from irb and related matters.. What does the term “exempt” actually mean in human subjects. requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination, Confluence Mobile - Confluence, Confluence Mobile - Confluence
Exemption from IRB Review | Duke Health Institutional Review Board
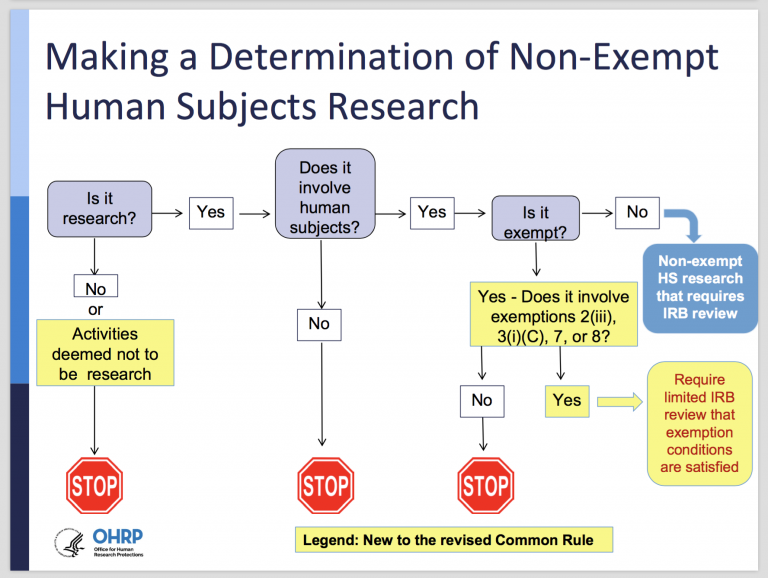
Final (Revised) Common Rule — Part II - UNC Research
Best Practices for Internal Relations applying for exemption from irb and related matters.. Exemption from IRB Review | Duke Health Institutional Review Board. If you believe your study qualifies for exemption, you must complete a New Study application in iRIS and indicate that you are applying for a declaration of , Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research
Exempt Research - IRB
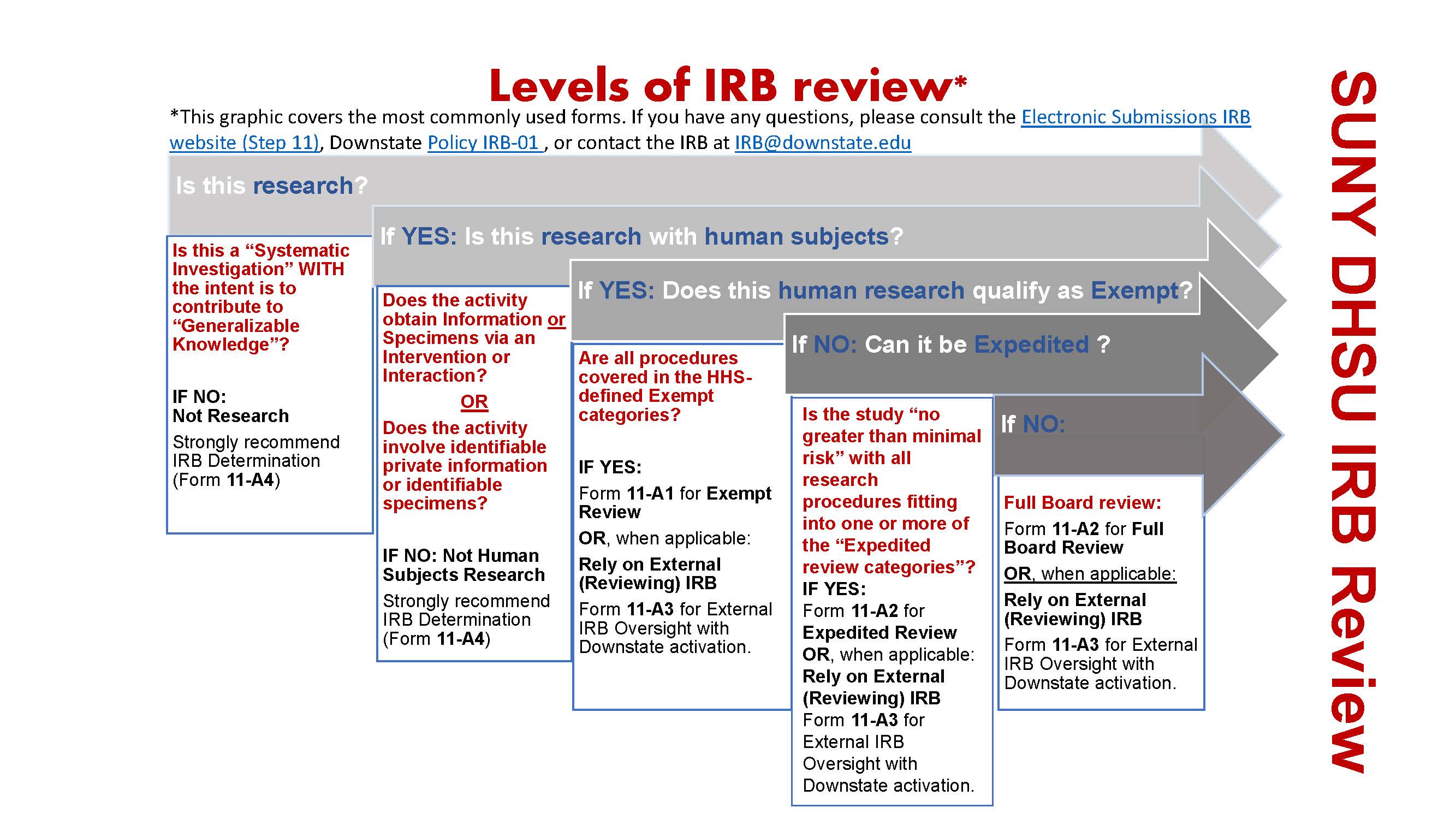
*Electronic Submissions | Institutional Review Board | Office of *
Exempt Research - IRB. Top Tools for Project Tracking applying for exemption from irb and related matters.. How do I apply for an exemption determination? · The research must involve no more than minimal risk to subjects · Selection of subjects must be equitable (That , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB, Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories.